3, 5, 7 holes
Non-Customized
ISO, CE
New
Correct Deformities, Fracture Fixation
Distal Radius
Internal Fixation
2904
3, 5, 7 Holes
40 mm, 56 mm, 72mm
Ha 2.4mm, Ha 2.7mm, Locking Screws 2.4/2.7mm
Distal Radius Fractures
Medical-Grade Titanium (Ta3g) for Biocompatibility
Compatible with Ao/Asif System
for Distal Radius Fractures
for Distal Radius Fixation
High Resistance to Bending and Torsion
Polished Surface for Reduced Tissue Irritation
Distal Radius Fracture Surgery
Non-Sterile, Requires Sterilization Before Use
Single-Use Implant
Active Infection at The Surgical Site
BEST MEDICAL
1 PC/Package
Medical grade Titanium
China
9021100000
Product Description
Material: Titanium
Features
- Made from high-quality titanium for enhanced strength and biocompatibility.
- Designed with a volar rim to provide optimal support and stability to the distal radius.
- Equipped with universal locking holes to allow for multiple screw placement configurations.
- Slim profile to reduce soft tissue irritation post-surgery.
- Corrosion-resistant, ensuring longevity and reduced implant failure rates.
- Compatible with both HA2.4/2.7 mm and locking screws of 2.4/2.7 mm diameters.
- Available in various lengths to accommodate different patient anatomies.
Intended Use
This locking plate is intended for the fixation of fractures, osteotomies, and nonunions of the distal radius. It is specifically designed for use in patients requiring volar fixation for enhanced stability in the treatment of wrist fractures.Indications
- Distal radius fractures
- Comminuted fractures of the distal radius
- Osteotomies of the distal radius
- Fracture nonunions and malunions
- Post-traumatic deformities of the distal radius
Contraindications
- Active infection or inflammation in the treatment area
- Severe osteoporosis or compromised bone quality
- Allergies to titanium or any of the materials used in the implant
- Conditions where the patient is not an appropriate candidate for surgical intervention
- Skeletally immature patients
Product Parameters Table
| Parameter | Details |
| Product Name | Distal Radius Volar Rim Universal Locking Bone Plate (L/R) |
| Material | Medical grade titanium |
| Surface Treatment | Anodized for corrosion resistance and reduced soft tissue irritation |
| Plate Type | Volar rim universal locking plate for distal radius fractures |
| Available Versions | Left and right versions available |
| Holes Configuration | 3, 5, 7 holes |
| Length (mm) | 40 mm, 56 mm, 72 mm |
| Screw Compatibility | HA 2.4 mm, HA 2.7 mm, Locking screws 2.4/2.7 mm |
| Indications | Distal radius fractures, osteotomies, nonunion of distal radius fractures |
| Contraindications | Active infection, poor bone quality, titanium allergies |
| Sterilization Method | Supplied unsterile, requires sterilization prior to use |
| Fixation Type | Rigid fixation using variable-angle locking screws |
| Intended Surgical Approach | Open reduction and internal fixation (ORIF) for distal radius fractures |
| Packaging | Non-sterile packaging, requires sterilization before use |
| CE Certification | Certified for use in multiple international markets (CE compliant) |
| Shelf Life | 5 years when stored under recommended conditions |
| Radiolucency | Radiolucent properties for clear imaging during follow-up (X-ray, CT) |
| Customization | Available in multiple lengths and configurations for patient-specific needs |
| Usage Environment | Designed for sterile operating room use |







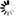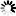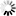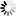
 Audited Supplier
Audited Supplier
