MY-PS
Biotechnology Industry, Chemical industry, Clinical Diagnosis, Food & Beverage Factory, Food Safety, Forensic Science, Pharmaceutical Industry
20000 L/hr
CE, FDA, GMP, ISO
Fully Automatic
Filtration, Ultrafiltration
1year
Stainless Steel 316L
150ºC
2~15000L
3%
380V 50Hz
IP65
Temperature Range-10~150ºC ±0.2ºC
Speed Range 0~450rpm ±3.0%
Pressure Range-0.01MPa~0.06MPa ±0.01MPa
Marya
Wooden Box
350*180*300
China
Product Description
Marya Intelligent Automation Formulation mixing tank System Pharmaceutical Preparation Systems Sterilization Grade Liquid Filtration
Description of the production steps of a general preparation system: Classification of the preparation system:
Classification of the preparation system:

















- Description of the preparation system:
Description of the production steps of a general preparation system:

- General preparation system: It is used for general small molecule chemical preparation, such as aqueous injection, lyophilized powder injection and so on.

2)Complex preparation system: It is used in the production of preparations with complex processes, such as suspensions, emulsions, liposomes, microspheres and other special preparations.

3)Biological preparation system: It is used for the preparation of all kinds of products applying biological engineering technology, such as the semi-finished preparation of antibodies, vaccines, blood products, recombinant proteins and so on, and it can also be used for the production of culture medium, purification buffer and other auxiliary liquid preparation.

4)CIP station system: It is used to assist in the on-line cleaning of tanks and pipelines of various preparation and bioreactor systems, and can be controlled separately or integrated with other systems.

- Performance Characteristics of the preparation system:
- The system can be fully automatic, with modular design, and its follow-up installation and maintenance is convenient.
- The automatic control system of CIP and SIP can be turned on by one key, which can automatically detect the end of cleaning and sterilization, with recipe management, electronic signature, electronic records, audit tracking and other computer functions.
- When the equipment is running, it can realize controllable production of key process parameters of drugs (such as temperature, dissolved oxygen, PH, etc.), and can automatically detect them and give an alarm when there are special conditions outside the set parameters, so as to avoid quality problems of drugs.
- The system follows ASME BPE and GMP design concepts, meets 3D blind corner requirements, and allows for low residue and continuous production.
- Before manufacturing the equipment in our factory, we will carry out one-to-one three-dimensional design to rationalize the arrangement of pipelines, valves, instruments, etc. in the system to make the operation of the equipment more user-friendly.
- Product Parameters
| Name | Parameters |
| Working volume | 2~15000L |
| Stirring speed control accuracy | Speed range 0~450rpm ±3.0% |
| Temperature control accuracy | Temperature range-10~150ºC ±0.2ºC |
| Pressure control accuracy | Pressure range-0.01Mpa~0.06Mpa ±0.01MPa |
| Weighing control accuracy | 3‰ |
| Power supply | 380V 50Hz (Note: Other voltages require a transformer) |
| Sealing and protection levels | IP65 |




















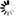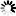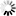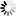
 Audited Supplier
Audited Supplier
